1. Why Cleanrooms Matter
Cleanrooms are not just shiny white rooms with people in bunny suits. They are mission-critical environments where the tiniest particle can make or break outcomes worth millions.
Think of a pharmaceutical lab: one speck of dust can contaminate a vaccine batch. Or a semiconductor plant: a single airborne fiber can ruin microchips before they ever leave the line.
In simple words: cleanrooms exist to control airborne particles. And who ensures this? The HVAC system. As engineers, our designs decide if a room stays at ISO Class 5 or slips into contamination. Airflow, pressure, temperature, and filtration — all fall under our responsibility.
Explore HVAC MEP design for more details. https://enershares.com/hvac-mep-thumb-rules/
This is why understanding ISO 14644 is not optional. It’s the baseline standard that every serious HVAC professional working with controlled environments must know.
2. What Is ISO 14644?
ISO 14644 is the global standard for cleanrooms and controlled environments. It replaced the old US Federal Standard 209E in 2001, bringing the world under one rulebook.
It classifies cleanrooms by the number of particles allowed per cubic meter of air. The stricter the class, the fewer particles you can have.
- ISO Class 5 → Extremely strict. Allows only 3,520 particles of size ≥0.5 microns per cubic meter. This is the standard for critical pharma filling lines or semiconductor wafer fabs.
- ISO Class 8 → Less strict. Allows 3,520,000 particles of the same size per cubic meter. Often used for less sensitive manufacturing areas.
Think of these classes as a “report card” for your cleanroom air quality. If your HVAC design doesn’t hit these numbers, your cleanroom fails.
ISO 14644-1 Classification (Particles ≥0.5 µm per cubic meter)
| ISO Class | Maximum Particles/m³ (≥0.5 µm) |
|---|---|
| ISO Class 5 | 3,520 |
| ISO Class 6 | 35,200 |
| ISO Class 7 | 352,000 |
| ISO Class 8 | 3,520,000 |
⚡ Enjoying this article? Stay updated with weekly HVAC & Energy Hacks — straight on WhatsApp!
💚 Join Our WhatsApp Channel3. From US Federal Standards to ISO – A Quick History
Old-timers in the industry will recall Federal Standard 209E, which used to classify cleanrooms as Class 100, Class 10,000, etc. That system was widely used in the US for decades.
But in 2001, 209E was withdrawn. Why? Because the world needed one global language for cleanrooms. That’s where ISO 14644 came in.
Today, whether you’re in the US, Europe, or Asia, when you talk about cleanroom classification, you talk ISO 14644.
4. US vs EU: ISO + Local Rules
Here’s where it gets interesting. While ISO 14644 is the baseline everywhere, different regions add their own compliance layers.
United States
- FDA (Food and Drug Administration) regulates pharmaceutical cleanrooms.
- They accept ISO 14644 classifications but tie them into GMP (Good Manufacturing Practice) rules.
- In addition, USP <797> and <800> define standards for sterile compounding pharmacies.
So if you’re designing or validating a pharma cleanroom in the US, you must meet ISO 14644 particle limits + FDA GMP requirements.
European Union
- The EU follows GMP Annex 1 for sterile drug manufacturing.
- Annex 1 uses Grades A, B, C, D for classification.
- These grades directly map to ISO 14644 particle counts. For example:
- Grade A ≈ ISO Class 5
- Grade B ≈ ISO Class 7 at rest
So again, ISO is the base, but Annex 1 adds its own layer of operational and monitoring rules.
5. Cleanroom HVAC: What Engineers Must Deliver
When it comes to HVAC design and operation for cleanrooms, ISO 14644 tells us the targets, but we must design the means to achieve them.
Key HVAC Responsibilities:
- Air Supply & Filtration
- Use HEPA filters for most classes, ULPA for very high requirements.
- Proper filter placement is critical: ceiling-mounted for vertical flow, wall-mounted for horizontal flow.
A diagram of laminar (unidirectional) airflow vs. turbulent (non-unidirectional) airflow, showing how air sweeps particles away in Class 5 areas. - Air Change Rates (ACH)
- Higher ISO classes demand more air changes per hour.
- Example: ISO Class 5 cleanrooms may require 240–600 ACH.
- Room Pressure Differentials
- Always keep cleaner areas at higher pressure than adjacent dirtier areas.
- This prevents backflow of contaminated air.
Illustrate pressure gradients from corridors (low) → Grade D → Grade C → Grade B → Grade A (high). - Temperature & Humidity Control
- Maintain narrow bands depending on product and process requirements.
- Some processes (like biotech) are highly sensitive to relative humidity.
- Validation & Monitoring
- Regular particle counts to ensure the system is delivering as per ISO 14644.
- Ongoing monitoring is mandatory for GMP environments.
| Parameter | Requirement / Guidance | Notes |
|---|---|---|
| Air Supply & Filtration | – HEPA filters for most classes (≥99.97% at 0.3 µm)- ULPA filters for critical zones (≥99.9995% at 0.12 µm)- Placement: Ceiling (vertical laminar flow), Wall (horizontal laminar flow) | 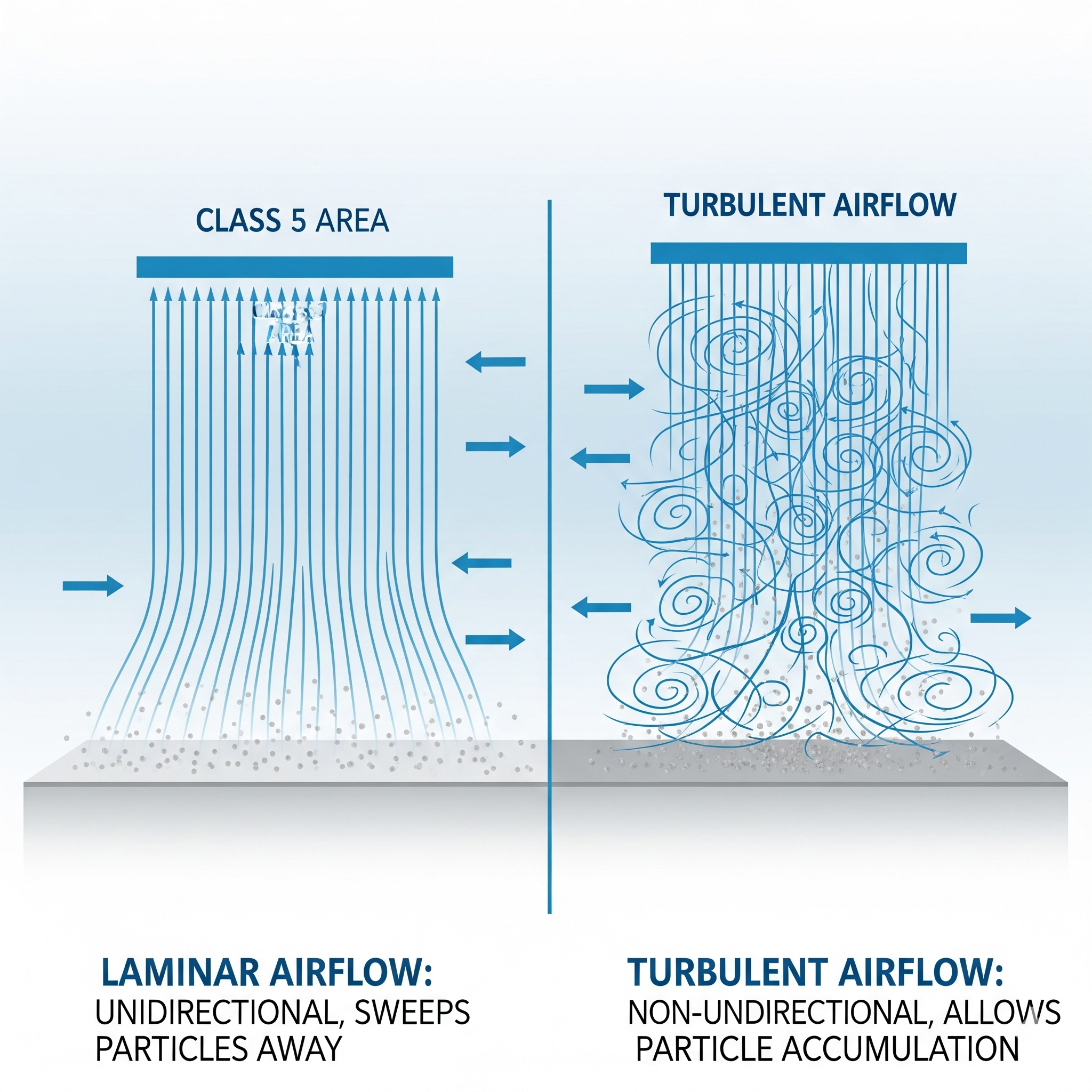 |
| Air Change Rates (ACH) | – ISO Class 5: 240–600 ACH- ISO Class 7–8: 30–60 ACH | Higher class → more ACH |
| Room Pressure Differentials | – Positive pressure from clean → less clean- Gradient: Corridor → ISO 8 → ISO 7 → ISO 6 → ISO 5 | 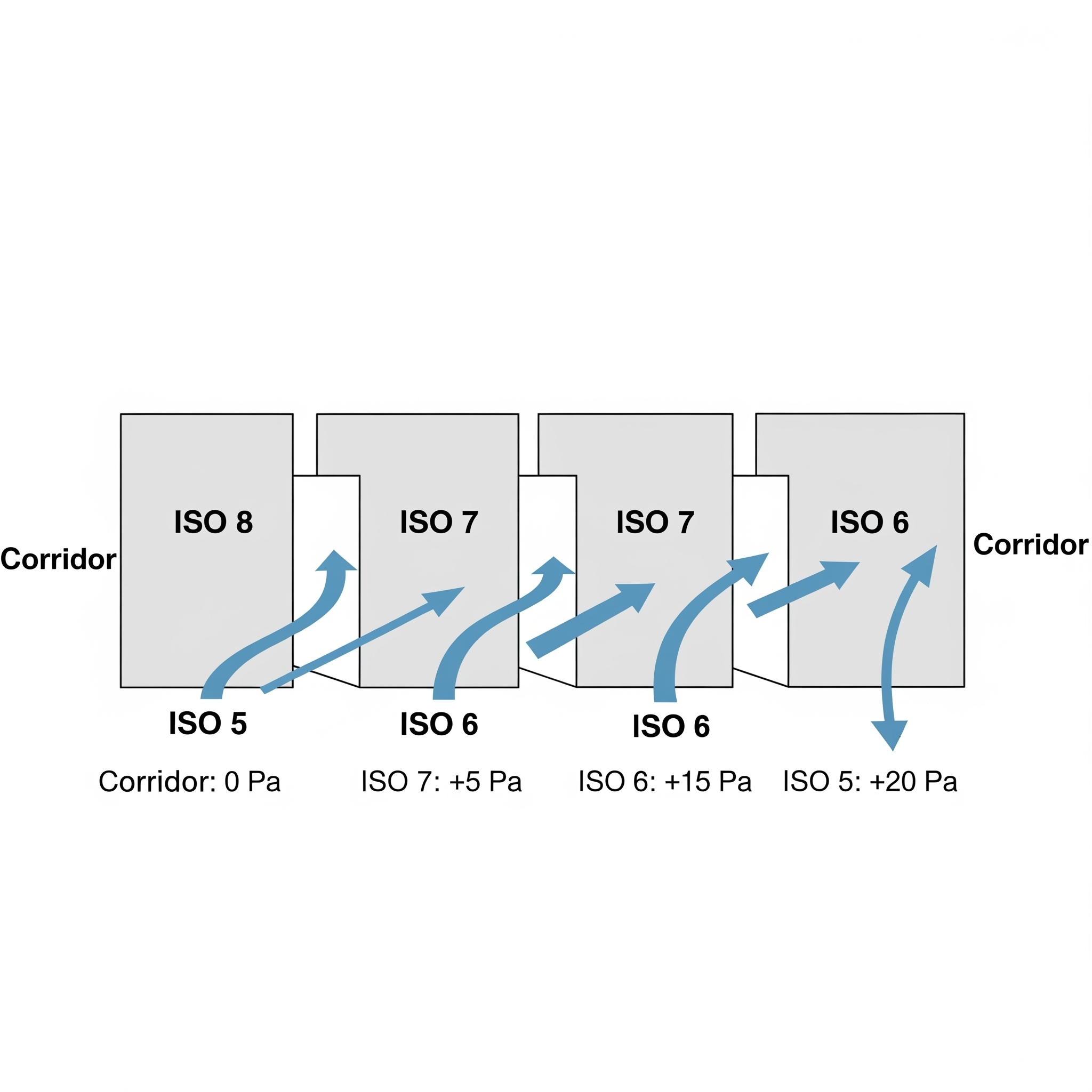 |
| Temperature & Humidity Control | – Typical: 20–22°C, RH: 40–60%- Adjust per process:• Electronics → low RH• Biotech → stable RH | Product/process specific |
| Validation & Monitoring | – Regular particle counts- Continuous monitoring in GMP areas- Data logging for audits | Ensures ISO 14644 + GMP compliance |
In short: we are not just supplying air, we are supplying compliance.
6. Common Missteps in Cleanroom HVAC
Even experienced engineers get caught in these traps:
- Overdesigning airflow – More air changes aren’t always better. It wastes energy and can disturb laminar flow.
- Ignoring pressurization cascades – Without proper pressure differentials, contamination creeps in.
- Poor filter maintenance – Even the best HEPA filter fails if seals are loose or replacement cycles are skipped.
- Not linking ISO with GMP – Meeting ISO counts alone is not enough for pharma. GMP rules demand procedural controls too.
Avoiding these mistakes saves clients from failed audits and costly downtime.
7. Mapping ISO 14644 to GMP Grades (Quick Reference)
Here’s a simplified mapping:
| EU GMP Grade | Typical Use | ISO 14644 Equivalent |
|---|---|---|
| A | Critical operations (filling, open vials) | ISO Class 5 |
| B | Background for Grade A | ISO Class 7 (at rest) |
| C | Less critical steps | ISO Class 8 |
| D | General clean areas | ISO Class 9 |
This table is a lifesaver when explaining cleanroom requirements to pharma clients.
8. Practical Example: Pharma Filling Line
Let’s make it practical.
A sterile filling line in the EU requires Grade A conditions. That means:
- ISO Class 5 particle count must be maintained at all times.
- Air is supplied through unidirectional HEPA filters.
- Surrounding background areas are Grade B (ISO Class 7).
- Pressure differential is at least 10–15 Pascals between Grade B and external corridors.
If your HVAC design cannot maintain this cascade, the filling line fails GMP inspection — even if your ISO particle counts were technically fine.
9. Looking Beyond ISO: Future Trends
Cleanroom standards keep evolving. A few trends every engineer should watch:
- Energy Efficiency in Cleanrooms: Cleanrooms are massive energy consumers. Smarter airflow design, VFD fans, and demand-based ventilation are becoming essential.
- Digital Monitoring: IoT sensors now track particles, pressure, and filter health in real-time.
- Exergy Analysis: Beyond energy efficiency, engineers are looking at exergy to optimize HVAC design.
- Hybrid Standards: Expect tighter integration of ISO 14644 with sustainability guidelines like ISO 50001 and green building codes.
10. Key Takeaway
Here’s the bottom line for every HVAC professional:
- ISO 14644 is the global baseline. If you talk cleanrooms, you talk ISO.
- US FDA and EU GMP add extra layers. Always cross-check local GMP rules.
- Your HVAC design decides compliance. Airflow, filters, pressure, and monitoring all rest on engineering decisions.
Think of yourself not as an HVAC designer, but as a guardian of product quality and patient safety. Because in cleanrooms, the air we supply is not just air — it’s compliance, quality, and trust.
Final Thought
Here’s the bottom line for every HVAC professional:
- ISO 14644 is the global baseline. If you talk cleanrooms, you talk ISO.
- US FDA and EU GMP add extra layers. Always cross-check local GMP rules.
- Your HVAC design decides compliance. Airflow, filters, pressure, and monitoring all rest on engineering decisions.
- Think of yourself not just as an HVAC designer, but as a guardian of product quality and patient safety. Because in cleanrooms, the air we supply isn’t just air—it’s compliance, quality, and trust.
A good engineer designs systems that move air. A great engineer designs systems that protect lives. Cleanroom HVAC is where that difference truly shows. And it all starts with understanding ISO 14644.


Nice one Mr. Anand
Insight full